PRR ADC02105-02151 - Monthly Compliance Rpts - 2013-09 - ASPC-Phoenix (redacted), AZ DOC, 2013
Download original document:
Document text

Document text
This text is machine-read, and may contain errors. Check the original document to verify accuracy.
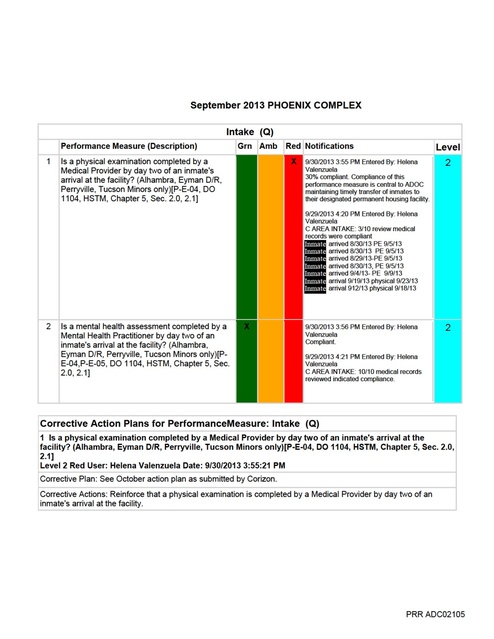
September 2013 PHOENIX COMPLEX Responsible Parties = FHA/DON/RDCQI/RVP Target Date-11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 3 Are vitals signs, to include weight, being checked and documented each time an inmate is seen during sick call? [P-E-04, HSTM Chapter 5, Section 1.3] Level 1 Amber User: Helena Valenzuela Date: 9/30/2013 3:29:19 PM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.In-service nursing staff on per Sick Call 2.20.2.2 contract performance outcome 3 (Sick Call Attachment); a.Agenda/sign off sheet to verify 2.Monitoring (Sick Call Monitoring Tool) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/RDCQI/RVP Target Date- 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 10/11/13 Update – VS will include weight when appropriate. 4 Is the SOAPE format being utilized in the inmate medical record for encounters? [DO 1104, HSTM Chapter 5, Section 1.3] Level 1 Red User: Helena Valenzuela Date: 9/30/2013 3:36:04 PM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.In-service all staff including providers on policy titled ”Continuous Progress Note (SOAP)”, Chapter 5, Section 1.3 (Attachment IV.1.) and per Sick Call 2.20.2.2 contract performance outcome 4 (Sick Call Attachment); use of Corizon NETs a.Agenda/sign off sheet to verify 2.Monitoring (Sick Call Monitoring Tool) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/Medical Director/RDCQI/RVP Target Date- 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 10/11/13 Update –NETs to be used for all Nursing sick call. PRR ADC02112 September 2013 PHOENIX COMPLEX Responsibile Parties = DON/Clinical Systems Business Analyst II/FHA/DON/RDCQI/RVP Target Date - 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 2 Are consultation reports being reviewed by the provider within seven (7) days of receipt? [CC 2.20.2.3] Level 2 Amber User: Vanessa Headstream Date: 9/27/2013 7:54:08 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized monitoring process 2.Communicate expectations via FHA/DON at quarterly training Regional office and obtain sign off sheet to verify 3.Monitoring (UM Audit Tool) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties =ARMD/RDON/RVP/RDCQI/DON/ Target Date-11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 2 Are consultation reports being reviewed by the provider within seven (7) days of receipt? [CC 2.20.2.3] Level 2 Amber User: Vanessa Headstream Date: 9/27/2013 7:54:08 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized monitoring process 2.Communicate expectations via FHA/DON at quarterly training Regional office and obtain sign off sheet to verify 3.Monitoring (UM Audit Tool) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties =ARMD/RDON/RVP/RDCQI/DON/ Target Date-11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. PRR ADC02115 September 2013 PHOENIX COMPLEX Responsible Parties = FHA/DON//Medical Director/RDCQI/RVP Target Date - 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 4 Have disease management guidelines been developed and implemented for Chronic Disease or other conditions not classified as CC? [P-G-01, HSTM Chpt. 5, Sec. 5.1, CC 2.20.2.4] Level 2 Amber User: Vanessa Headstream Date: 9/27/2013 8:10:20 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.In-service staff on Corizon Clinical Guidelines (I. – IV. Chronic Care Attachment) a.Agenda/sign off sheet to verify, inclusive of all pertinent staff 2.Monitoring a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/Medical Director/RDCQI/RVP Target Date- 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 10/11/13 Update – Make sure guidelines available at sites; need to prep chart for clinic visit so everything the provider needs is available. PRR ADC02120 September 2013 PHOENIX COMPLEX Corrective Actions: Reinforce with nursing staff the importance of using name stamps. Continue to monitor PRR ADC02125 September 2013 PHOENIX COMPLEX h.Practitioner Cards (Appendis I.1.h.) i.Controlled Medications (Appendix I.1.i.) 2.In-service staff a.Using information from 8/19 - 11/13 Regional office mandatory in-service and PharmaCorr policy b.Agenda/sign off sheet to verify, inclusive of all pertinent staff (Appendix I.2.b.) 3.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/IC/RDCQI/RVP Target Date-11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 10/11/13 Update – Statewide in Sept Redbook and MAR audit, results reviewed; to audit pharmacy in October related to Controlled Substances and Expired meds. 4 When a medication error occurs, is nursing staff completing a medication error report, documenting per policy and notifying Nursing Supervisor, Facility Health Administrator, who will notify all other Program Managers and ADC On-site Monitor? [HSTM Chapter 5, Section 6.6] Level 2 Amber User: Martin Winland Date: 9/30/2013 3:26:19 PM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized process statewide to include, but not limited to : a.Medication error documentation/reporting (Pharmacy Appendix). 2.In-service staff on process and PharmaCorr policy. a.Agenda/sign off sheet to verify, inclusive of all pertinent staff. 3.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed. b.Weekly site results discussed with RVP. c.Audit results discussed a monthly CQI meeting. d.Minutes and audit reported monthly to Regional office for tracking and trending. Responsible Parties =FHA/DON/RDCQI/RVP/FHA Target Date- 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. PRR ADC02127 September 2013 PHOENIX COMPLEX 6 Are reentry/discharge plans established no later than 30 days prior release for all inmates with a MH score of MH-3 and above? [CC 2.20.2.10] Level 2 Amber User: Nicole Taylor Date: 9/30/2013 2:28:09 PM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.In-service staff on process expectations per Mental Health 2.20.2.10 contract performance outcome 7 (Mental Health Attachment) related to re-entry plan a.SMI patients will be followed by discharge planners utilizing the data from the SMI monthly report tool; MH3 patients will be given community resources by MH Clinicians and documented in the chart; all patients receiving psychotropic medications will be seen by Psychiatrist/Psychiatry CNP b.Agenda/sign off sheet to verify, inclusive of all pertinent staff 2.Monitoring (Mental Health Monitoring Tool) a.Audit tools developed b.Monthly site results discussed with RVP/MH Director c.Audit results discussed at monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Regional office for tracking and trending Responsible Parties = FHA/DON/Mental Health Director/RVP/RDON/RDCQI/MH Lead Target Date- 11/30/13 Continue to monitor monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. PRR ADC02131 September 2013 PHOENIX COMPLEX Corrective Action Plans for PerformanceMeasure: Medication Administration 4 Are the Medication Administration Records (MAR) being completed in accordance with standard nursing practices? [HSTM Chapter 4, Section 1.1, Chapter 5, Section 6.4] Level 1 Amber User: Vanessa Headstream Date: 9/27/2013 8:19:38 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized process statewide to include, but not limited to : a.Refusals/No Show - Policy titled “Appointment or Treatment Refusals” Chapter 5, Section 7.2 (Appendix VI.1.a.). b.MAR documentation. c.Administration of DOT/KOP. d.Printing MARs (Pharmacy Appendix). e.Medication error documentation/reporting (Pharmacy Appendix). 2.In-service staff on process and PharmaCorr policy. a.Agenda/sign off sheet to verify, inclusive of all pertinent staff. 3.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed. b.Weekly site results discussed with RVP. c.Audit results discussed a monthly CQI meeting. d.Minutes and audit reported monthly to Regional office for tracking and trending. Responsible Parties =FHA/DON/RDCQI/RVP/FHA Target Date- 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 5 Are medication errors forwarded to the FHA to review corrective action plan? Level 2 Amber User: Vanessa Headstream Date: 9/30/2013 1:55:38 PM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized process statewide to include, but not limited to : a.Medication error documentation/reporting (Pharmacy Appendix). 2.In-service staff on process and PharmaCorr policy. a.Agenda/sign off sheet to verify, inclusive of all pertinent staff. 3.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed. b.Weekly site results discussed with RVP. c.Audit results discussed a monthly CQI meeting. d.Minutes and audit reported monthly to Regional office for tracking and trending. Responsible Parties =FHA/DON/RDCQI/RVP/FHA Target Date- 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 6 Are there any unreasonable delays in inmate receiving prescribed medications? Level 2 Red User: Vanessa Headstream Date: 9/27/2013 8:27:30 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by CorizonIntakes1.Standardized process for meds to be available to inmate upon transfer (Pharmacy Appendix 1 & 2) a.Intake Orders b.Private Prisons 2.In-service staff on process per PharmaCorr policy, a.Agenda/sign off sheet to verify, inclusive of all pertinent staff 3.Custody educated regarding contract requirements regarding inmate transfer with meds. 4.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting PRR ADC02141 September 2013 PHOENIX COMPLEX d.Minutes and audit reported monthly to Regional office for tracking and trending Responsibile Parties = FHA/DON/Custody/RDCQI/RVP Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results 1.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending 2.Standardized process statewide to include, but not limited to (Appendix III.1.): a.Internal b.External 2.In-service staff on process and ADC policy titled “Continuity of Care Upon Transfer” Chapter 5, Section 5.0 (Appendices III.2.); a.Agenda/sign off sheet to verify, inclusive of all pertinent staff 3.Custody educated regarding contract requirements regarding inmate transfer with meds 4.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/Custody/RDCQI/RVP Target Date - 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 8 Are chronic condition medication expiration dates being reviewed prior to expiration to ensure continuity of care? [NCCHC Standard P-D-01] Level 2 Red User: Vanessa Headstream Date: 9/27/2013 8:28:55 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized process for meds to be available to inmate upon transfer (Pharmacy Appendix 1 & 2) 2.In-service staff on process per PharmaCorr policy, a.Agenda/sign off sheet to verify, inclusive of all pertinent staff 3.Custody educated regarding contract requirements regarding inmate transfer with meds. 4.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsibile Parties = FHA/DON/Custody/RDCQI/RVP Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results 1.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending 2.Standardized process statewide to include, but not limited to (Appendix III.1.): a.Internal b.External 2.In-service staff on process and ADC policy titled “Continuity of Care Upon Transfer” Chapter 5, Section 5.0 (Appendices III.2.); a.Agenda/sign off sheet to verify, inclusive of all pertinent staff 3.Custody educated regarding contract requirements regarding inmate transfer with meds 4.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP PRR ADC02142 September 2013 PHOENIX COMPLEX c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/Custody/RDCQI/RVP Target Date - 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 9 Are non-formulary requests being reviewed for approval or disapproval within 24 to 48 hours? Level 2 Amber User: Vanessa Headstream Date: 9/27/2013 8:31:58 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized process statewide, to include but not limited to (Pharmacy Appendix 1 & 2): a.Non-formulary process (Appendix I.1.d.) i.Reviewed for approval within 24-48 hrs ii.Providers notified decision within 24-48 hrs e.Manifest Reconciliation f.Inventory control g.Stock Medications h.Practitioner Cards (Appendis I.1.h.) i.Controlled Medications (Appendix I.1.i.) 2.In-service staff a.Using information from 8/19 - 11/13 Regional office mandatory in-service and PharmaCorr policy b.Agenda/sign off sheet to verify, inclusive of all pertinent staff (Appendix I.2.b.) 3.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/IC/RDCQI/RVP Target Date-11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 10/11/13 Update – Statewide in Sept Redbook and MAR audit, results reviewed; to audit pharmacy in October related to Controlled Substances and Expired meds. 10 Are providers being notified of non-formulary decisions within 24 to 48 hours? Level 2 Amber User: Vanessa Headstream Date: 9/27/2013 8:32:57 AM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized process statewide, to include but not limited to (Pharmacy Appendix 1 & 2): a.Non-formulary process (Appendix I.1.d.) i.Reviewed for approval within 24-48 hrs ii.Providers notified decision within 24-48 hrs e.Manifest Reconciliation f.Inventory control g.Stock Medications h.Practitioner Cards (Appendis I.1.h.) i.Controlled Medications (Appendix I.1.i.) 2.In-service staff a.Using information from 8/19 - 11/13 Regional office mandatory in-service and PharmaCorr policy b.Agenda/sign off sheet to verify, inclusive of all pertinent staff (Appendix I.2.b.) 3.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed b.Weekly site results discussed with RVP c.Audit results discussed a monthly CQI meeting d.Minutes and audit reported monthly to Regional office for tracking and trending Responsible Parties = FHA/DON/IC/RDCQI/RVP PRR ADC02143 September 2013 PHOENIX COMPLEX Target Date-11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. 10/11/13 Update – Statewide in Sept Redbook and MAR audit, results reviewed; to audit pharmacy in October related to Controlled Substances and Expired meds. 11 Are medication error reports being completed and medication errors documented? Level 2 Amber User: Vanessa Headstream Date: 9/30/2013 1:56:54 PM Corrective Plan: See October action plan as submitted by Corizon. Corrective Actions: October Action plan submitted by Corizon1.Standardized process statewide to include, but not limited to : a.Medication error documentation/reporting (Pharmacy Appendix). 2.In-service staff on process and PharmaCorr policy. a.Agenda/sign off sheet to verify, inclusive of all pertinent staff. 3.Monitoring (Appendix I. - IV Monitoring Tools) a.Audit tools developed. b.Weekly site results discussed with RVP. c.Audit results discussed a monthly CQI meeting. d.Minutes and audit reported monthly to Regional office for tracking and trending. Responsible Parties =FHA/DON/RDCQI/RVP/FHA Target Date- 11/30/13 Continue to monitor weekly x 3 weeks, monthly until within compliance, then quarterly; monitoring frequency using audit tool per audit results. PRR ADC02144

